JEE Mains 2025 PYQ – Heat and Thermodynamics | 22nd Jan Shift 1
Question:
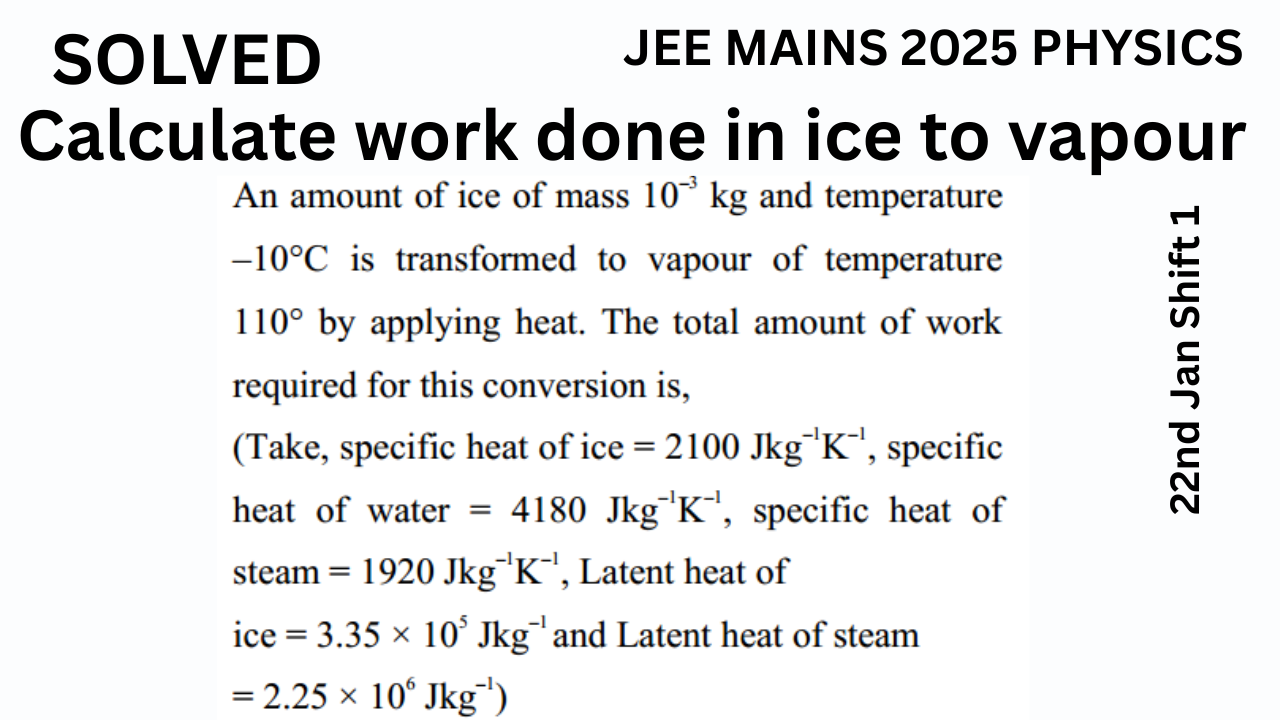
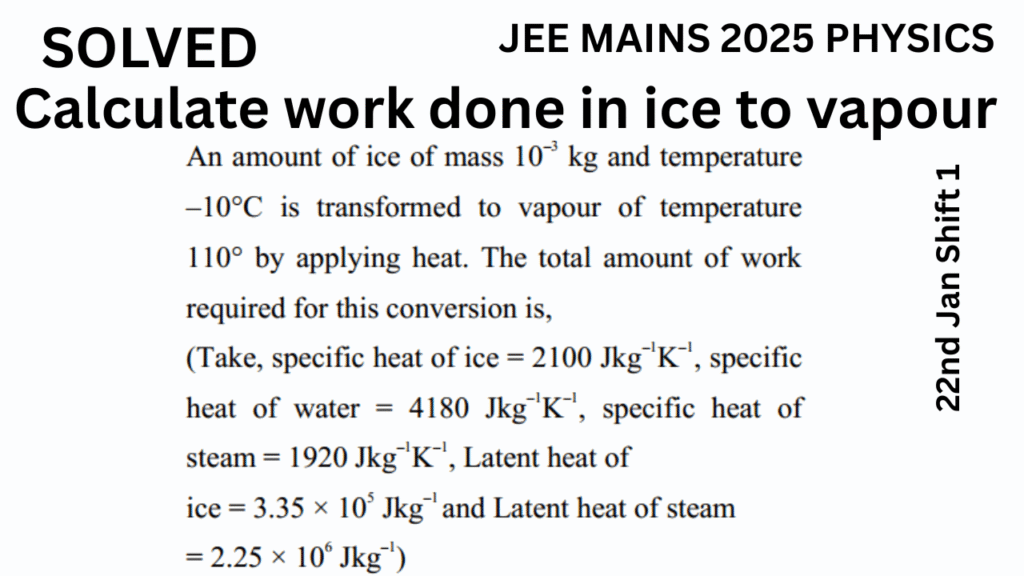
An amount of ice of mass \(10^{-3} \, \text{kg}\) and temperature –10°C is transformed to vapour of temperature 110°C by applying heat. The total amount of work required for this conversion is:
Given:
- Specific heat of ice = \(2100 \, \text{J/kg·K}\)
- Specific heat of water = \(4180 \, \text{J/kg·K}\)
- Specific heat of steam = \(1920 \, \text{J/kg·K}\)
- Latent heat of fusion of ice = \(3.35 \times 10^5 \, \text{J/kg}\)
- Latent heat of vaporization = \(2.25 \times 10^6 \, \text{J/kg}\)
Step-by-Step Solution:
1. Heating ice from -10°C to 0°C:
Heat required, \(Q_1 = m \times c_{\text{ice}} \times \Delta T\)
\(= (10^{-3}) \times 2100 \times 10 = 21 \, \text{J}\)
2. Melting the ice at 0°C:
Heat required, \(Q_2 = m \times L_{\text{fusion}}\)
\(= (10^{-3}) \times 3.35 \times 10^5 = 335 \, \text{J}\)
3. Heating water from 0°C to 100°C:
Heat required, \(Q_3 = m \times c_{\text{water}} \times \Delta T\)
\(= (10^{-3}) \times 4180 \times 100 = 418 \, \text{J}\)
4. Vaporizing water at 100°C:
Heat required, \(Q_4 = m \times L_{\text{vap}}\)
\(= (10^{-3}) \times 2.25 \times 10^6 = 2250 \, \text{J}\)
5. Heating steam from 100°C to 110°C:
Heat required, \(Q_5 = m \times c_{\text{steam}} \times \Delta T\)
\(= (10^{-3}) \times 1920 \times 10 = 19.2 \, \text{J}\)
Total Heat Required:
\[
Q_{\text{total}} = Q_1 + Q_2 + Q_3 + Q_4 + Q_5 = 21 + 335 + 418 + 2250 + 19.2 = 3043.2 \, \text{J}
\]
✅ Final Answer: (2) 3043 J
Why This Question Is Important:
- This is a JEE Mains 2025 Heat and Thermodynamics PYQ which Combines concepts of specific heat, latent heat, and phase change
- Tests multi-step understanding of energy transformations
- Relevant for both JEE Mains 2026 and JEE Mains 2027
📹 Watch Full Video Explanation:
🔗 Follow Us for More PYQs and Concepts:
- 🌐 Website: Solved Insights
- 📘 Facebook: Follow us
- 📸 Instagram: @solvedinsights
- 📺 YouTube: Solved Insights Channel
- 📱 WhatsApp Group: Join Now
- 📱 Telegram Group: Join Now